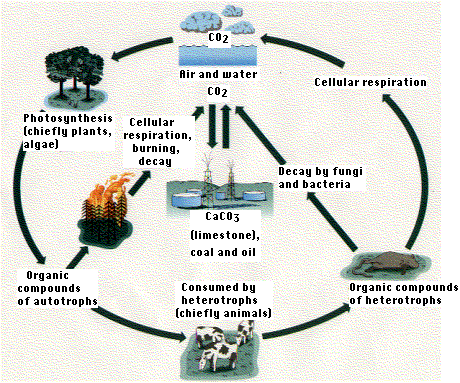
The Carbon Cycle - The concentration of carbon in living matter (18%) is almost 100 times greater than its concentration in the earth (0.19%). So living things extract carbon from their nonliving environment. For life to continue, this carbon must be recycled. That is our topic.
How to Get Carbon
Carbon exists in the nonliving environment as:
- carbon dioxide in the atmosphere and dissolved in water (forming HCO3-)
- carbonate rocks (limestone and coral - CaCO3)
- deposits of coal, petroleum, and natural gas derived from once-living things
- dead organic matter, e.g., humus in the soil
How Does Carbon Enters the World?
Carbon enters the biotic world through the action of autotrophs:
primarily photoautotrophs, like plants and algae, that use the energy of light to convert carbon dioxide to organic matter.
and to a small extent, chemoautotrophs - bacteria and archaeans that do the same but use the energy derived from an oxidation of molecules in their substrate.
Carbon returns to the atmosphere and water by
- respiration (as CO2)
- burning
- decay (producing CO2 if oxygen is present, methane (CH4) if it is not.
The uptake and return of CO2 are not in balance
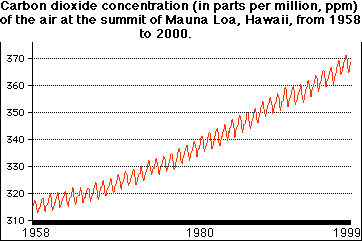
The carbon dioxide content of the atmosphere is gradually and steadily increasing. The graph shows the CO2 concentration at the summit of Mauna Loa in Hawaii from 1958 through 1999. The values are in parts per million (ppm). The seasonal fluctuation is caused by the increased uptake of CO2 by plants in the summer.
The increase in CO2 probably began with the start of the industrial revolution. Samples of air trapped over the centuries in the glacial ice of Greenland show no change in CO2 content until 300 years ago.
Since measurements of atmospheric CO2 began late in the nineteenth century, its concentration has risen over 20%. This increase is surely "anthropogenic"; that is, caused by human activities:
- burning fossil fuels (coal, oil, natural gas) which returns to the atmosphere carbon that has been locked within the earth for millions of years.
- clearing and burning of forests, especially in the tropics. In recent decades, large areas of the Amazon rainforest have been cleared for agriculture and cattle grazing.
Where is the missing carbon?
Curiously, the increase in atmospheric CO2 is only about one-half of what would have been expected from the amount of fossil fuel consumption and forest burning.
Where has the rest gone?
Research has shown that increased CO2 levels lead to increased net production by photoautotrophs. There is some evidence that the missing CO2 has been incorporated by
- Increased growth of forests, esp. in North America
- Increased amounts of phytoplankton in the oceans.
The Greenhouse Effect and Global Warming
Despite these "sinks" for our greatly increased CO2 production, the concentration of atmospheric CO2 continues to rise? Should we be worried?
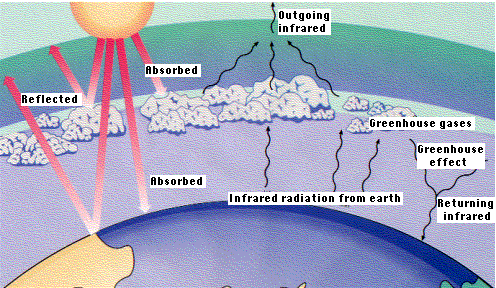
Carbon dioxide is transparent to light but rather opaque to heat rays. Therefore, CO2 in the atmosphere retards the radiation of heat from the earth back into space - the "greenhouse effect".
Will the increase in carbon dioxide lead to global warming?
At this point, the answer depends on limited data and what assumptions you plug into your computer models. The very activities that deposit CO2 in the air also deposit dust particles that impede the passage of light through the atmosphere so more is reflected back into space. Air pollution thus appears to reduce the arrival of energy as well as its departure. Whether these opposing effects cancel each other out is not yet known.
Average temperatures do seem to have increased slightly in recent decades.
Some evidence:
- Careful monitoring of both ocean and land temperatures.
- Many glaciers and ice sheets are receding.
- Woody shrubs are now growing in areas of northern Alaska that 50 years ago were barren tundra.
- Many species of birds and butterflies are moving north and breeding earlier in the spring.
But throughout its history the earth has gone through many periods of climatic change (e.g., ice ages). Whether the present change is a result of human activities or of long-term changes over which we have no control is still uncertain. But a developing consensus is telling us that it is better to be safe than sorry.
READ ALSO: 7 Tips On How to Speed Up Slow Internet on Your Android Device
Other Greenhouse Gases
Although their levels in the atmosphere are much lower than that of CO2, methane (CH4) and chlorofluorocarbons (CFCs) are also potent greenhouse gases.
Methane
Although methane ("marsh gas") is released by natural processes, human activities may now account for over one-half of the total.
- growing rice in paddies
- burning forests
- raising cattle (fermentation in their rumens produces methane that is expelled - collectively adding an estimated 100 million tons a year to the atmosphere). (But estimates can be wrong. In 1990, the U.S. Environmental Protection Agency estimated that rice paddies were also adding about 100 million tons a year; accurate measurements later showed that this estimate was ten times too high.)
So burning of the tropical rainforest produces a double whammy: incomplete combustion during burning releases large amounts of methane as do the cattle that are later placed on the cleared land.
The methane concentration in the air is presently some 1700 parts per billion (ppb) and is growing at a rate of 1% per year. Although this concentration is far less than that of CO2, methane is 30 times as potent a greenhouse gas and so may now be responsible for 15-20% of the predicted global warming.
The marked warming of the earth that occurred at the end of the Paleocene epoch is thought to have been caused by the release of large amounts of methane from the sea floor.
Chlorofluorocarbons (CFCs)
Chlorofluorocarbons (CFCs) are synthetic gases in which the hydrogen atoms of methane are replaced by atoms of fluorine and chlorine (e.g., CHF2Cl, CFCl3, CF2Cl2).These gases are non inflammable, nontoxic, and very stable. They are widely used in industry as:
- refrigerants (e.g., in refrigerators and air conditioners)
- solvents
- propellants in aerosol cans (now banned in some countries)
- in the manufacture of plastic foams.
They escape to the air from all of these uses (e.g., from leaky and discarded refrigeration units).
Their chemical inertness, which makes CFCs so desirable for industry, also makes them a threat to the atmosphere. Once in the atmosphere, it may take 60-100 years for them to decompose and disappear. In the meantime, they may contribute to as much as 25% of the greenhouse effect. But perhaps even more worrisome is the threat they pose to the ozone shield.
Do you suppose Carbon Cycle are viable? Sympathetically share your remarks with us by reaching us through the comment section or on our contact page.